Why do Researchers Need Volunteers for COVID-19 Vaccine Trial?
The need for a volunteer for the novel coronavirus vaccine trial indicates that other than a miracle, a tested vaccine is our most potent weapon to dig ourselves out of the pandemic. Through the researchers' efforts to design, test, and soon, mass-produce a vaccine that will bring an immunity against COVID-19, the hopes never stop.
According to HuffPost, there was initial evidence on the effectiveness and safety of the potential vaccines. Dr. Anthony Fauci, Director of the National Institute for Allergy and Infectious Diseases, stated that vaccines are something we need to be "cautiously optimistic" about.
However, there is no assurance that any of the vaccines currently being tested will do the trick. Vaccines undergo more clinical trials not because scientists want, but because it is needed. And when you go through a trial of the vaccine, there should be volunteers. These volunteers are from different ages, genders, ethnicities, and backgrounds. The category of volunteers is needed to ensure that the vaccine works across all and any general population.
How is it to be a volunteer for the COVID-19 vaccine trials?

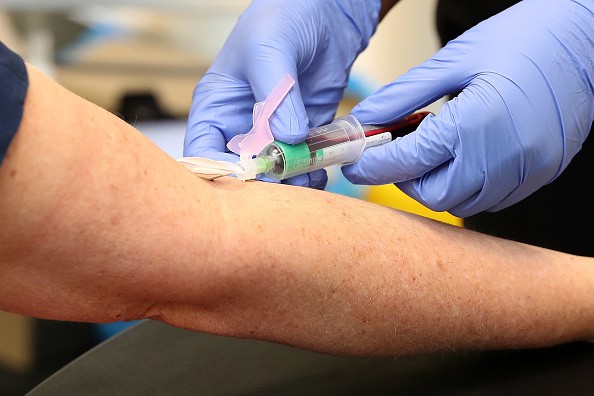
Jim Kublin, executive director of the operations program for the National Institutes of Health-funded COVID-19 Prevention Network (CoVPN) and an infectious disease researcher at the Fred Hutchinson Cancer Research Center, explained that there are screening to be a volunteer for a vaccine trial to ensure that you are in good health. The first screening is through phone, and if you pass, you will then proceed to an in-person screening, which would include a physical. If you pass, it is a green light for you to have blood drawn and receive the first dose of the vaccine through injection.
After having the first dose, you will need to record any symptoms or side effects that you will encounter. The observation will go on for a few weeks. Then you will go back to the trial site to have a quick health exam, have more blood drawn, and take a second dose, which boosts the vaccine's effect. The same tracking of symptoms will be implied for a few more weeks.
You will be required for a quick blood draw every three to six months for up to two years to check if your immune response holds up or wanes unless you have symptoms or side effects that will require further evaluation. The frequency of blood draws on what the researchers are looking.
The vaccine is made to turn the immune system and go after the virus if you were exposed. But keep in mind that vaccines cannot give you coronavirus as these vaccines don't contain the virus.
The senior vice president for clinical affairs and ambulatory care at NYU Langone Andrew Rubin participates in a Phase 1 trial. The process has been relatively simple as per Rubin. The first appointment took three hours, and the subsequent appointment only lasted for half an hour.
Rubin got involved in the vaccine trial because, according to him, he wanted to give back to all the health care workers who have been sacrificing and battling COVID-19. "I wanted to be able to say I contributed to the effort to stop this crisis," Rubin said.
Volunteers get compensated, here's how to sign up!
Kublin said that volunteers for the COVID-19 vaccine trials get compensated for their time. Most tests pay about $50 per appointment, which can have a total of $500 to $1200 for the full trial.
In the previous report of LatinPost on how to register as volunteer for the vaccine trial, here are additional details.
If you want to sign up and become a vaccine testing volunteer, there are some qualifying factors. First, you must be at least 18 years old and do not breastfeed or is not pregnant. Trials may also be risky for people who had been severely immunocompromised or those who have cancer. Vaccine trials are not allowed those who have a history of being allergic to vaccines and won't be able to take contraceptives during the trial.
If you are planning to enroll, you could read more details about the clinical trials for a COVID-19 vaccine. It may be helpful for you to review the initial testing results.
Meanwhile, the easiest way to sign up is through the CoVPN's website, which is now recruiting people for the Phase 3 trials. Meanwhile, to know the nearest trials in your area, visit www.CovidStudies.org.
Check these out:
New Study Claims The Taller You Are, The Higher Risk of Getting COVID-19
Manufactured Antibodies May Be Next Big COVID-19 Treatment
COVID-19 Eye Transmission: Should We All Be Wearing Goggles or Face Shields?
Subscribe to Latin Post!
Sign up for our free newsletter for the Latest coverage!













