Your Questions About COVID-19 Vaccine Answered: Side Effects, Costs, and More
The COVID-19 vaccine has been something the world is looking forward to for months and questions about its side effects, costs and others are starting to rise as drug makers start seeing promising results.
According to USA Today, a COVID-19 vaccine might reach some Americans by year's end.
Pfizer and Moderna already filed for emergency use authorization from the Food and Drug Administration. As these vaccines are still awaiting distribution, what do we know about these shots? What are the side effects?
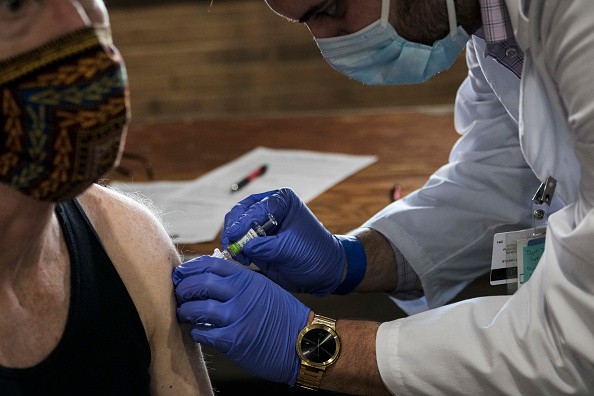
Experts answered some of the world's frequently asked questions about the COVID-19 vaccines. Here's what we know so far:
Is There a COVID-19 Vaccine in the U.S.?
There is no authorized COVID-19 vaccine in the U.S. yet. However, several companies have already opened distribution.
As per the FDA's rules, a vaccine can't be sent to administration sites until it gets their stamp of approval.
As said by the Centers for Disease Control and Prevention (CDC), the government's Operation Warp Speed has a goal to deliver only safe and effective vaccines to the general public.
So no vaccine will be administered in the country until there is approval. But Warp Speed also plans to begin vaccine deliveries within 24 hours of approval from the FDA.
Does Russia or China Have COVID-19 Vaccines Already?
According to BBC, Russia already started its COVID-19 vaccination program starting with the most at-risk populations.
Sputnik V, the country's vaccine candidate was said to be 95% effective and showed no major side effects, but it has not yet finished its final trial stages.
Meanwhile, provincial governments in China already placed orders for experimental and domestic vaccines, but these inoculations haven't been fully proven to help against the coronavirus, reported Bloomberg.
Developers are said to be speeding up the final testing as they try to overtake Pfizer's run for emergency approval.
What Are the Possible Side Effects of a COVID-19 Vaccine?
According to Dr. C. Buddy Creech, director of the Vanderbilt Vaccine Research Program, vaccine side effects usually start showing up to two months after getting vaccinated.
He told Market Watch these side effects usually include fevers, headaches and feeling run down.
But USA Today added that the country's leading candidate vaccine usually report mild to moderate side effects like pain at the injection site, fatigue, and aching muscles and joints for a day or two.
How Much Would the Vaccine Cost? Will Insurance Cover It?
According to CDC, vaccine doses bought with taxpayer money will be given to Americans for free.
Moderna and Pfizer already pledged with some 100 million doses that will be funded through federal support.
But the CDC warned that some vaccine providers will charge administration fees for giving the shot to someone.
"Vaccine providers can get this fee reimbursed by the patient's public or private insurance company or, for uninsured patients, by the Health Resources and Services Administration's Provider Relief Fund," the agency added.
How Long Can a COVID-19 Vaccine Protect Me?
Even though the vaccine have shown efficacy against the virus, there is still limited data available to say for how long the protection could last.
"That protection may wane over time, and you may be susceptible again," Dr. William Schaffner, professor of health policy and of preventive medicine at the Vanderbilt University School of Medicine, told USA Today.
It is also unknown whether the vaccine will trigger different immune responses across different groups of people.
Subscribe to Latin Post!
Sign up for our free newsletter for the Latest coverage!
















