Regeneron Stops Trial of Antibody Drug for Severely Ill COVID-19 Patients
Regeneron Pharmaceuticals has paused a clinical trial of its experimental antibody drug for seriously ill COVID-19 patients because of a potential safety concern, The Hill reported.
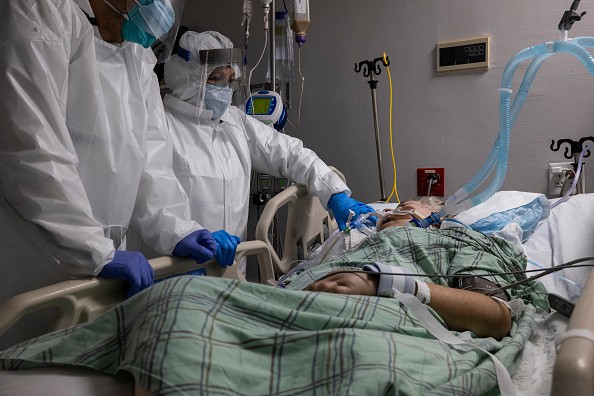
On Friday, Regeneron said it is halting the enrollment of very sick patients who receive its advanced COVID-19 treatment in a trial testing based on an independent safety board's recommendation.
The company said the affected patients are those receiving mechanical ventilation or high levels of oxygen.
The independent monitoring committee noted that it observed "a potential safety signal and unfavorable risk/benefit profile at this time."
The committee further suggested collecting additional data on already enrolled patients. It also recommended maintaining trial enrollment of hospitalized patients that need little or no oxygen.
In consideration for emergency use authorization in mild-to-moderate outpatients at high risk for poor outcomes, the halt does not affect other trials or studies of Regeneron's antibody drug.
Regeneron earlier said a separate study definitively showed a remarkable reduction in viral load and the need for more medical visits.
A different monitoring committee has recommended stopping enrollment in a study testing of Eli Lilly antibody drug to probe a possible safety issue in hospitalized patients.
The National Institute of Allergy and Infectious Diseases (NIAID), which funded the study, announced Monday that there's no longer a safety issue, but the trial had to stop due to the board's finding that it had a little clinical benefit in the treatment.
There are no antibody drugs yet that have been authorized for use, but Regeneron's antibody treatment recently made headlines after President Donald Trump received it and touted it as a "miracle" drug.
There is no cure yet for COVID-19, but treatments are improving, and the available treatments depend on the severity of COVID-19 patients' infection.
Dr. Anthony Fauci, director of NIAID, said that treatment is all about timing. Fauci added that dexamethasone and other steroids, as well as antiviral drug remdesivir, could help sickest patients on ventilation.
Meanwhile, studies with experimental antibody drugs suggest they are most effective when given outpatient care to someone with mild symptoms.
Spread of COVID-19 on household members is 'common'
According to CNN, a new study from the U.S. Centers for Disease Control and Prevention (CDC) claims that the spread of coronavirus among family members after one person becomes infected is "common" and occurs quickly.
The study published in the CDC's Morbidity and Mortality Weekly Report on Friday said that the person suspected or exposed to having COVID-19 must isolate before getting tested to protect other family members.
A CDC-led team of researchers said: "Because prompt isolation of individual with COVID-19 can reduce household transmission, suspected persons that they might have coronavirus should isolate, stay at home, and use a separate bathroom and bedroom if possible."
Check these out:
Mouthwash Could Inactivate Spread of Human Coronavirus, Study Says
Hispanic Women More Vulnerable to COVID-19 During Pregnancy, Study Finds
COVID-19 Patients: 80% of Them Are Vitamin D Deficient, Study Claims
Subscribe to Latin Post!
Sign up for our free newsletter for the Latest coverage!















